Go back
Alzheimer’s Disease Anti-Amyloid Immunotherapies (ARIA) 3.0T
Patient information
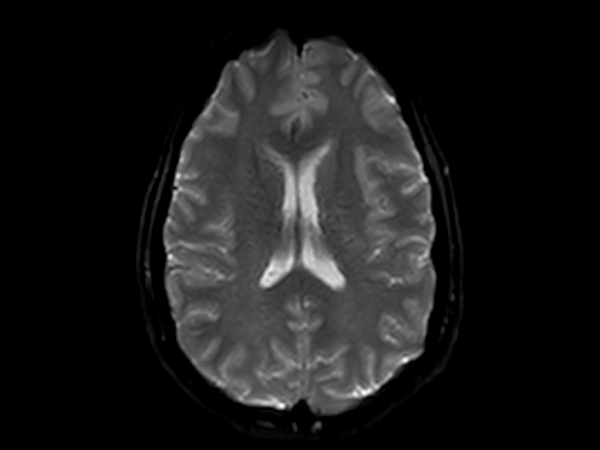
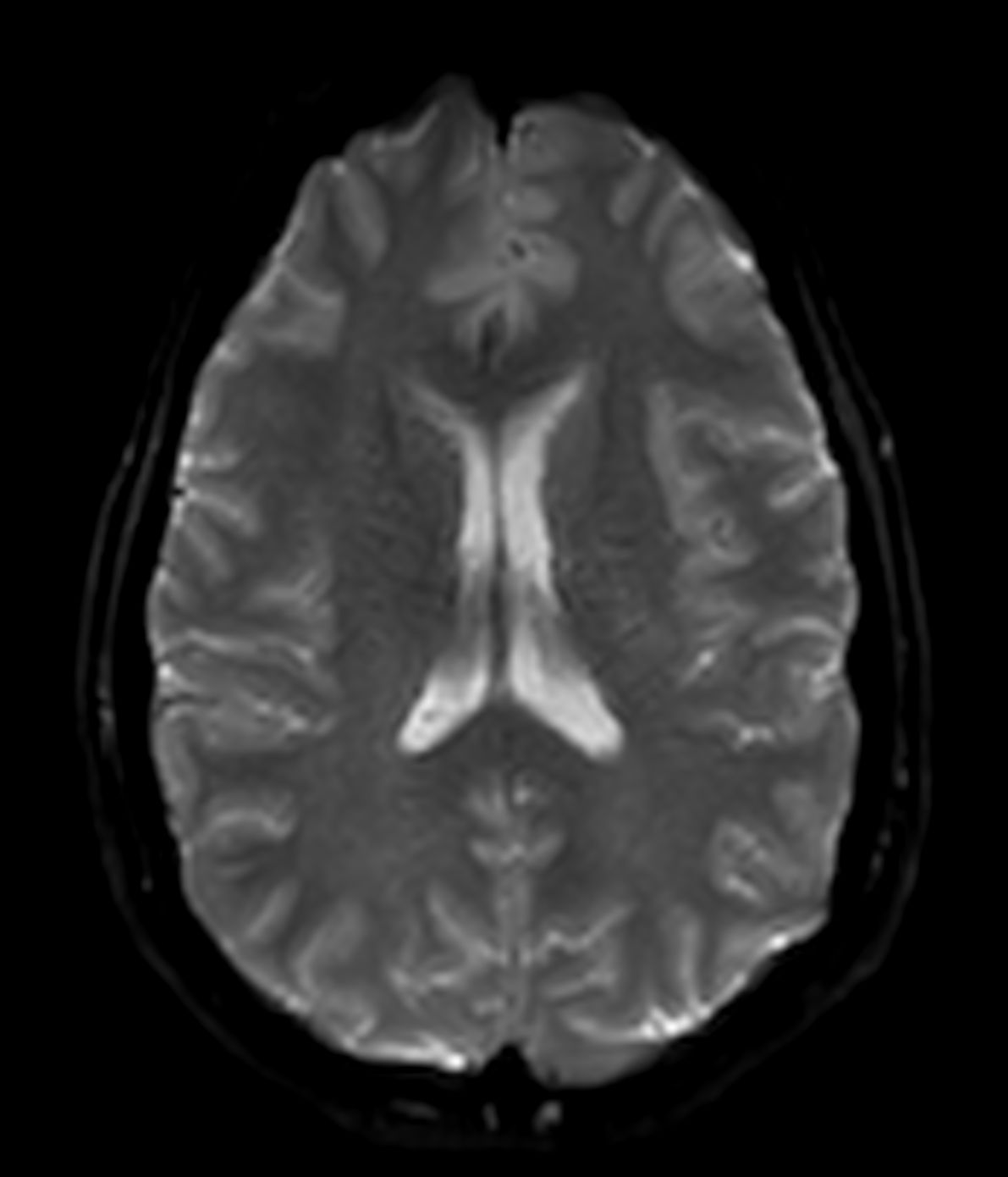
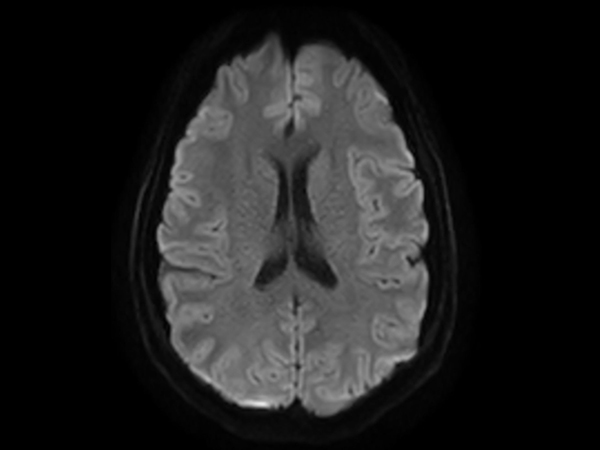
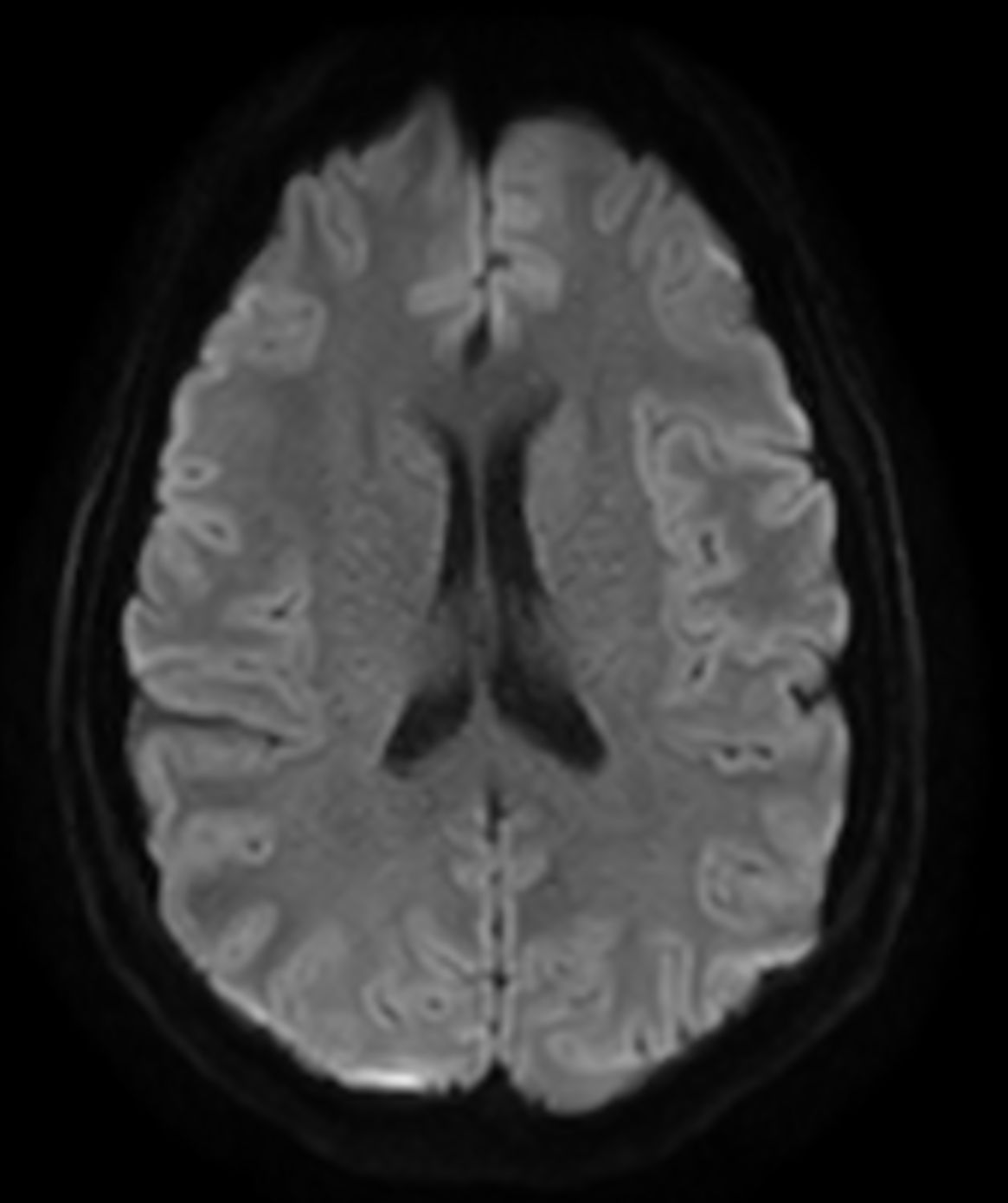
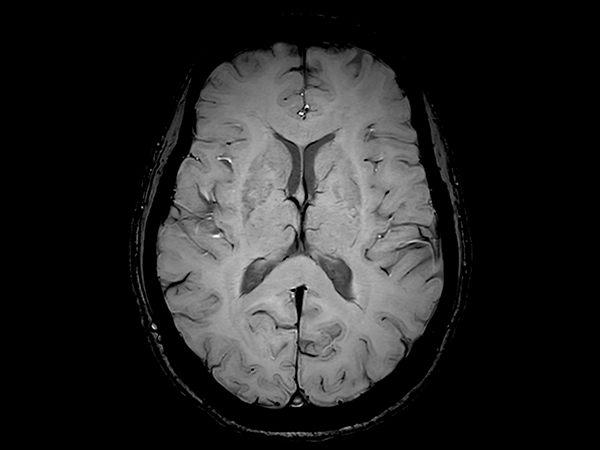
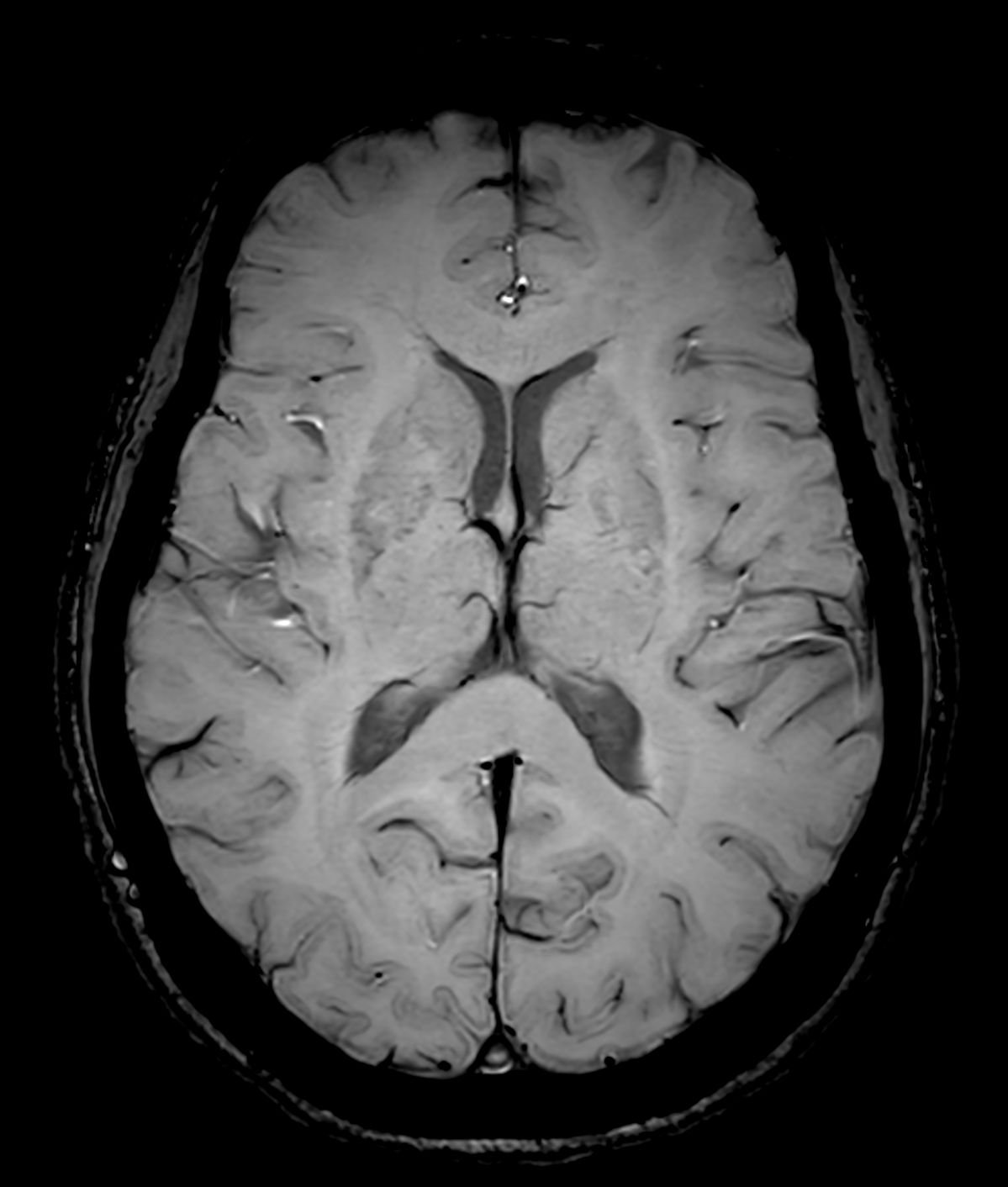
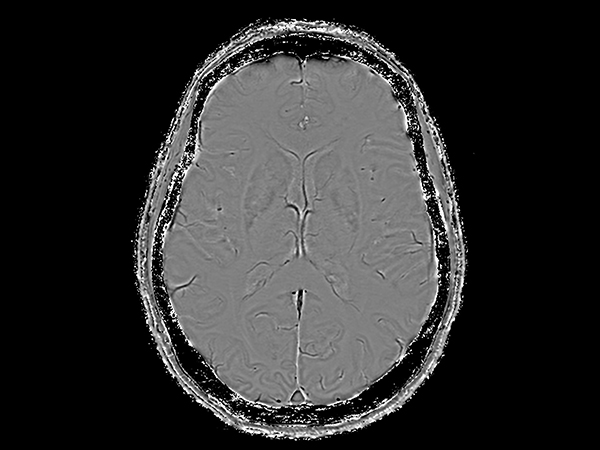
Amyloid clearing medication, such as Aduhelm (Aducanumab) and Leqembi (Lecanemab) have been cleared by the FDA in 2022/2023, to slow down cognitive decline in early-stage Alzheimer’s disease. ASNR-recommendations for AD therapeutic imaging were published in 2022 for eligibility assessment as well as for monitoring for amyloid-related imaging abnormalities. This ExamCard includes ASNR-recommended consensus protocols for imaging of Alzheimer’s Disease Anti-Amyloid Immunotherapies (ARIA). (Cogswell et al., AJNR 2022,43(9)E19-E35;DOI: https://doi.org/10.3174/ajnr.A7586))Gallery
More Information
*Results from case studies are not predictive of results in other cases. Results in other cases may vary.